Computer System Validation In Pharma Industry
Computer system validation in pharma industry. In the US the Food and Drug Administration is the main regulating and controlling body. A pharma business manager needs to keep in mind that CSV is an industry standard accepted on the international level. Computer System Validation CSV is a documented process required by regulatory agencies to verify that a computerized system does precisely what its designed to do.
It also involves careful planning of various stages in the qualification and validation of applicationsoftware used in the manufacturing process and all the work should be carried out in a structured way according to standardized working procedures. This guide aims to suggest the tools and strategies necessary and appropriate for use in the validation of computerized systems for human and veterinary Pharmaceutical industries Pharmaceutical chemicals APIS and excipients Biologics Biotechnology Blood Products Gas Medicinal Products and medical Devices used in activities. Its essential to maintain quality standards in pharma since non-conformance can have far-reaching consequences.
FDA 21 CFR part 1110 c. Computer System Validation in the Pharma Industry. Quality Transparency Innovation.
With our collective industry and regulatory knowledge our teams offer tailored services for Computer System Validation to deliver the outcomes your company needs to be successful in computerized system lifecycle management. What is Computer System Validation The purpose of the validation process is to provide a high degree of assurance that a specific process or in this case computer system will consistently produce a product control information or data which meets predetermined specifications and quality attributes. Whether it is Computer System Validation or Process Validation or Equipment Validation the intent of Validation is to ensure that a systemprocess meets its intended use and can deliver consistent and reliable results over its life cycle.
However most validation approaches in regulated Pharmaceutical Medical Devices and Biotech companies still. There are multiple types of validation in the pharmaceutical such as Analytical Method Validation Process Validation Cleaning Validation Equipment Validation HVAC System Validation Facility Validation and Computer System Validation. PharmaState DNA is having four pillars.
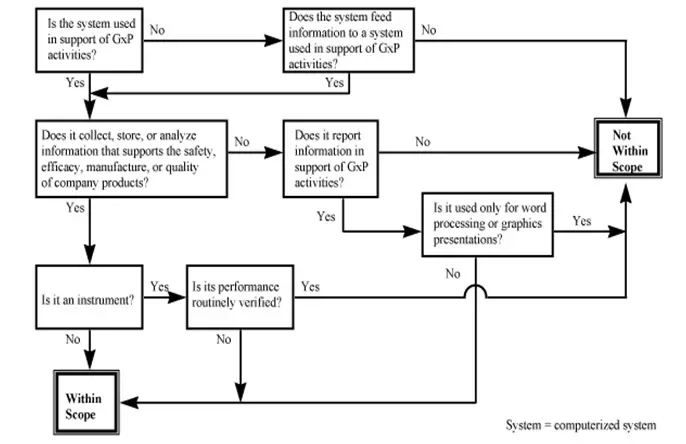
This includes manufacturing production inventory management packaging and labeling and maintenance processes and functionality. Computerized systems are increasingly becoming the norm across every industry including pharmaceuticals. In every industry validation is plays an important role to produce results in a consistent manner.
These validation activities and results shall be documented. All software changes shall be validated before approval and issuance.
FDA 21 CFR part 1110 c.
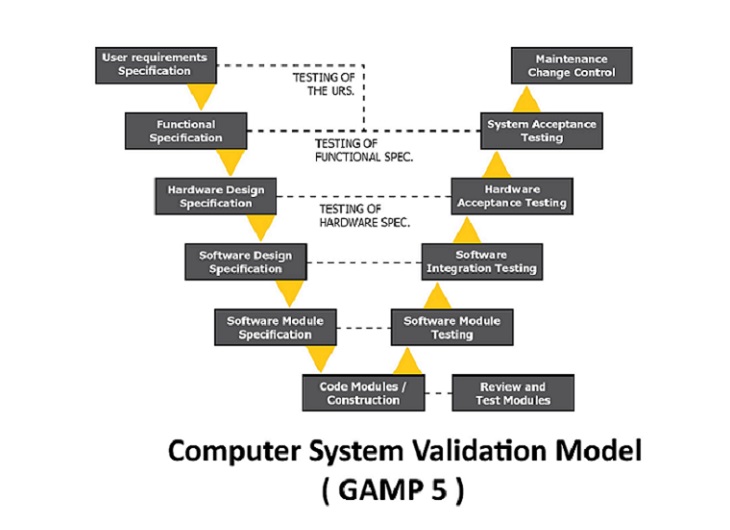
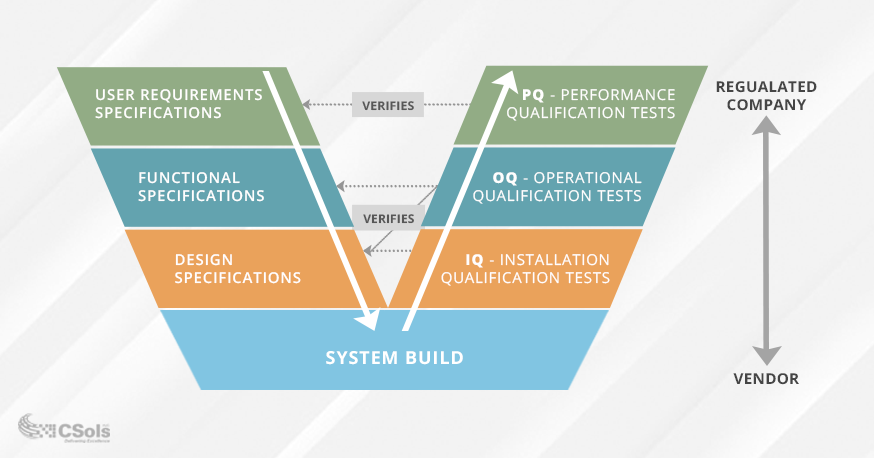
Pharm Res 2020 44. Copyright Hesham A and Patan IK. Data that meet a. FDA 21 CFR part 11 d. As such computer system validation CSV has become an important part of pharmaceutical cGMP often provided along with qualification and calibration services by accredited labs or compliance service providers. The computerized system which are adapted in manufacturing of pharmaceuticals is tested for their worthiness if the actual readings obtained from system and those obtained in manually in process are both matching and are accurate and satisfactory so as to rely completely on the computer systems adapted in the process of pharmaceutical manufacturing and quality control and quality. There are multiple types of validation in the pharmaceutical such as Analytical Method Validation Process Validation Cleaning Validation Equipment Validation HVAC System Validation Facility Validation and Computer System Validation. Cannabis growers and producers should validate any software system that might affect the quality of their product according to GMP standards. These validation activities and results shall be documented.
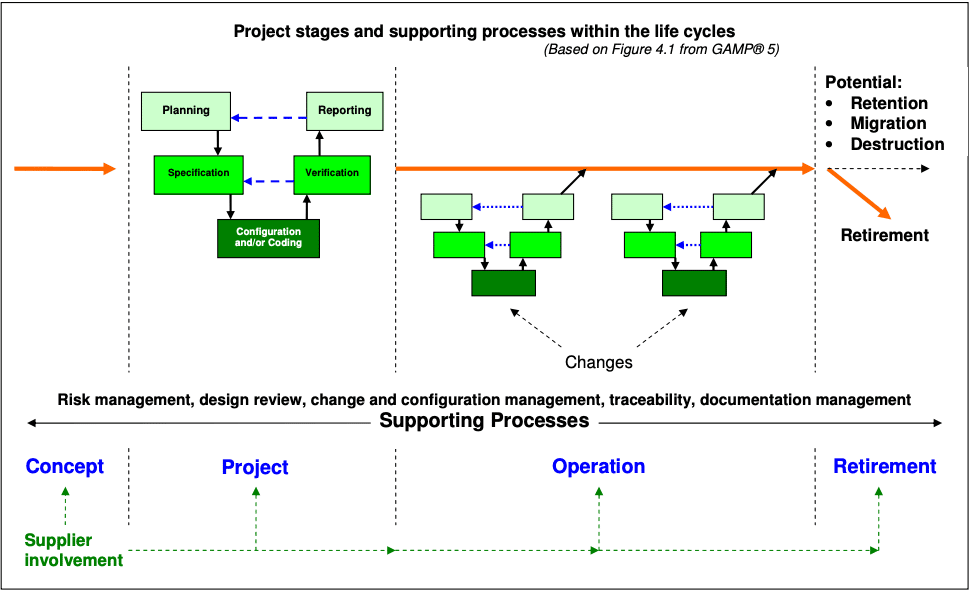
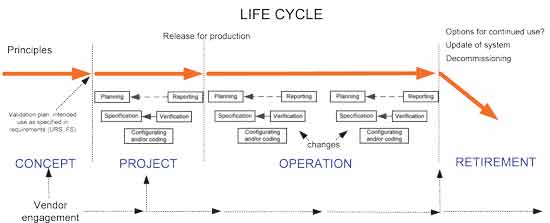
Hesham A and Patan IK. Data that meet a. Computer system validation is a necessity in the pharma industry to ensure adherence to pharmaceutical cGMP guidelines and to help companies maintain consistent quality. Computer system validation involves a series of activities that are taking place during the life cycle of processes. Validation of computer system shall be carried out to ensure that all computer systems within the organization are developed installed and implemented in a systematic way performing as intended and to ensure that the systems are being maintained in a state of control throughout the life cycle in compliance with applicable GxP regulations. Quality Transparency Innovation. All software changes shall be validated before approval and issuance.































Post a Comment for "Computer System Validation In Pharma Industry"